
Solutions are homogeneous systems thatcontain two or more components, as well as products that are the result of the interaction of these components. They can be in a solid, liquid or gaseous state. Let us consider the liquid aggregate state of solutions. Their composition includes a solvent and a substance dissolved in it (the latter is less).
The colligative properties of solutions are theirCharacteristics that are directly dependent only on the solvent and the concentration of the solution. They are also called collective or collective. The colligative properties of solutions appear in mixtures in which there is no chemical interaction between the constituent components. In addition, the forces of mutual action between the particles of the solvent and the particles of the solvent and the substance dissolved in it are equal in ideal solutions.
Colligative properties of solutions:
1) The vapor pressure is lower over the solution than over the solvent.
2) Crystallization of the solution occurs at a temperature below the crystallization temperature of the solvent in its pure form.
3) The boiling of the solution occurs at a higher temperature than the boiling of the solvent itself.
4) The phenomenon of osmosis.

Consider the colligative properties separately.
Equilibrium at the boundary of phases in a closed system:Liquid vapor is characterized by saturated vapor pressure. Since in the solution part of the surface layer is filled with molecules of the solute, equilibrium will be achieved at a lower vapor pressure.
The second colligative property is the decreasethe crystallization temperature of the solution as compared with the solvent is due to the fact that the particles of the dissolved substance interfere in the construction of the crystals and, thus, prevent crystallization with decreasing temperature.
The boiling point of the mixture is higher than the solvent inpure form, due to the fact that the equality of the atmospheric pressure and the pressure of saturated steam is achieved with greater heating, since some of the molecules of the solvent are associated with the particles of the dissolved substance.

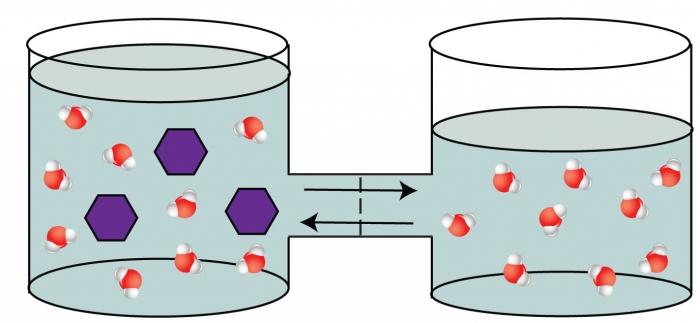
The fourth collusive property of solutions is the phenomenon of osmosis.
The phenomenon of osmosis is the ability of the solventmigrate through a septum that is permeable to some particles (solvent molecules) and impenetrable to others (matter molecules dissolved). This septum separates the solution with a high content of solute from a less concentrated solution. An example of such a semipermeable partition can serve as a membrane of a living cell, a bovine bubble, etc. The phenomenon of osmosis is caused by equalization of concentrations on both sides, separated by a membrane, which is thermodynamically more beneficial for the system. As a result of the displacement of the solvent into a more concentrated solution, an increase in pressure is observed in this part of the vessel. This overpressure was called osmotic.
Mathematically, the colligative properties of solutions of nonelectrolytes can be represented by the equations:
Δ Tkip = Sketch ∙ Sm;
Δ Tcr. = Kzam ∙ Sm;
π = CRT.
Coligative properties in numerical termsdiffer for solutions of electrolytes and solutions of nonelectrolytes. For the first, they are somewhat larger. This is due to the fact that they are electrolytic dissociation, and the number of particles is significantly increased.
The colligative properties of solutions are wideuse in the home and at work, for example, the phenomenon of osmosis is used to produce clean water. In living organisms many systems are also built on the colligative properties of solutions (for example, plant cell growth).


























