
The largest and most diverse amongInorganic substances is a class of complex compounds. The group of organometallic substances, such as chlorophyll and hemoglobin, can be attributed to it. It is these compounds that are the bridge that combines inorganic and organic chemistry into a single science. The role of complex substances in the development of knowledge in the field of analytical chemistry and crystal chemistry, in the study of the most important biological processes: photosynthesis, internal (cellular) respiration, is invaluable.

In this article, we will study the structure and nomenclature of complex compounds, as well as the basic principles of their classification.
At the end of the 20th century, the Swiss scientist A.Werner proved that there are several structures in the molecule of any complex substance, which were respectively called the central ion, ligands (addends) and the outer coordination sphere. So that the classification and nomenclature of complex compounds become clear to us, let us examine these concepts in more detail. So, A. Werner proved the presence in the molecule of an ion (usually positively charged), occupying a central position. It became known as the complexing agent, central ion or atom. Near it can be located both neutral molecules, called ligands, and negatively charged anion particles, which form the internal coordination sphere of a substance. All remaining particles that are not included in it form the outer shell of the molecule.

So, in the formula of sodium cuprite Na2[Cu (OH)4], the central copper atom in the oxidation state +2 and four hydroxogroups constitute the inner sphere, and sodium ions are located at some distance from the central atom in the outer sphere.
So far, the theory of A.Werner remains the main theoretical base, on the basis of which complex complex compounds are studied. The nomenclature, that is, the names of these substances, are determined according to the rules adopted by the International Society of Pure and Applied Chemistry.
Let us give some examples of formulas of substances in which the complexing agent is represented by a platinum atom - K2[PtCl6] or NH molecules3 - [Ag (NH3)2Cl.As it turned out, the formulas can be derived using the following practical methods: double exchange reactions, on the molar conductivity of solutions, X-ray structural method. Consider these methods in more detail.
The substances of this group are characterized by the presence in the molecule of the central atom of platinum. If the compound is PtCl4× 6NH3 act silver nitrate solution thenall the chlorine present in the substance is bound to the metal atoms and white flakes of AgCl are formed. This means that all the chlorine anions were located in the outer coordination sphere, whereas ammonia molecules were bound to the central platinum atom and together with it formed the inner sphere.

Hence, the coordination formula of the substance will be written in this form: [Pt (NH3)6Cl4 and called hexammine platinum chloride. Using the X-ray structural method, chemists studied other complex compounds, the nomenclature of which will be established by us in the next section.
The structure of substances of this group was determined withusing the physical X-ray diffraction process underlying the X-ray analysis. Passing through the crystal lattice, electromagnetic waves are scattered by the action of electrons of the test substance. This makes it possible to very accurately determine which groups of atoms are in the lattice sites. For chromium-containing crystals, a corresponding nomenclature of complex compounds was created. Examples of the names of isomeric hydrates of trivalent chromium salts, prepared using the X-ray structural method, are the following: tetraacvadichlorochrome (III) chloride, pentaacachlorochrome (III) chloride.
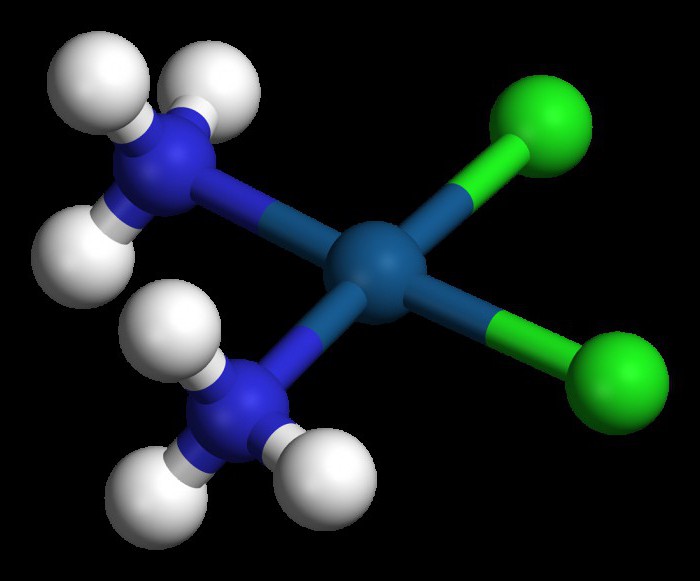
It was found that in these substances the chromium atom is associated with six different add-ons. How does this indicator determine and what factor influences the coordination number?
To answer the question above,Recall that in the immediate vicinity of the complexing agent are several structures called addends or ligands. Their total number determines the coordination number. According to the theory of A. Werner, the receipt, classification and nomenclature of complex compounds directly depend on this indicator. It is also correlatively related to the degree of oxidation of the central atom. In platinum, chromium, and iron compounds, the coordination number is most often six; if the complexing agent is represented by copper or zinc atoms - four, if the central atom is silver or copper - to two.
Chemistry distinguishes both main classes andtransitional series of substances between them. The complex compounds considered in the previous subtitles, the nomenclature of which indicates the presence of water molecules in their structure, belong to aquacomplexes. For ammoniata include substances containing neutral particles of ammonia, for example, triamminrodium triiodide. The class of chelate compounds is peculiar in structure of molecules. Their name comes from the biological term chelicera - the so-called claws of decapod crayfish. These substances contain addends, the spatial configuration of which covers the complexing agent, like claws. These compounds include oxalate complex of ferric iron, ethylene diammine complex of platinum with oxidation state +4, salts of aminoacetic acid, which include rhodium, platinum or copper ions.

The most common security question inassignments in chemistry in the course of high school sounds like this: name complex compounds according to the IUPAC nomenclature. For a specific example, let us analyze the algorithm for compiling the name of a substance having the following formula: (NH4)2[Pt (OH)2Sl4n.
As a result, the substance will have a name in which all of the above structures are listed.

At the beginning of the article we called the most importantrepresentatives of organometallic substances, such as hemoglobin, chlorophyll, vitamins. They play a leading role in metabolism. Complex compounds are widely used in technological cycles of ferrous and non-ferrous metals smelting. Carbonyls play an important role in metallurgy — special complex compounds whose nomenclature indicates the presence of carbon monoxide CO in their molecules in the form of addendum. These compounds decompose when heated and restore metals such as nickel, iron, cobalt from their ores. Most of the complex compounds are also used as catalysts in the reactions of obtaining varnishes, paints and plastics.


























