
Modern realities suggest a broadoperation of heat engines. Numerous attempts to replace them with electric motors have been failing so far. The problems associated with the accumulation of electricity in autonomous systems are being solved with great difficulty.
The problems of technology are still relevantmanufacture of electric energy accumulators taking into account their long-term use. The speed characteristics of electric vehicles are far from those of cars on internal combustion engines.
The first steps to create hybrid engines can significantly reduce harmful emissions in megacities, solving environmental problems.
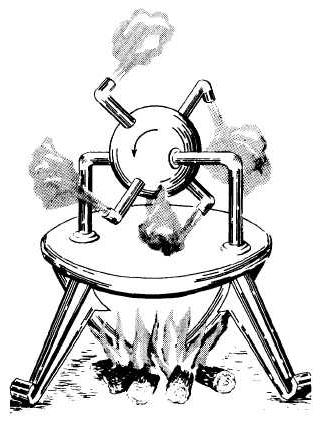
The possibility of converting steam energy into energymovement was known in ancient times. 130 BC: Philosopher Heron of Alexandria presented a steam toy - eolipil - to the audience. The sphere, filled with steam, came into rotation under the action of the jets emanating from it. This prototype of modern steam turbines in those days did not find application.
For many years and centuries the development of the philosopher was considered only an amusing toy. In 1629 the Italian D. Branci created an active turbine. Steam set in motion a disc equipped with blades.
From this moment the rapid development of steam engines began.

The transformation of the internal energy of fuel into the energy of movement of parts of machines and mechanisms is used in thermal machines.
The main parts of the machines are a heater (a system for obtaining energy from outside), a working medium (it makes a useful effect), a refrigerator.
The heater is designed for the working body to accumulate a sufficient supply of internal energy for committing useful work. The refrigerator takes away excess energy.
The main characteristic of efficiency is calledThermal efficiency. This value shows how much energy spent on heating is spent on committing useful work. The higher the efficiency, the better the performance of the machine, but this value can not exceed 100%.
Let the heater acquire from outside the energy equal to Q1. The working body did work A, while the energy given to the refrigerator was Q2.
Based on the definition, calculate the value of the efficiency:
η = A / Q1. We take into account that A = Q1 - Q2.
Hence the efficiency of a thermal machine, the formula of which has the form η = (Q1 - Q2) / Q1 = 1 - Q2/ Q1, allows us to draw the following conclusions:

Is it possible to create such an engine,whose efficiency would be maximum (ideally - 100%)? A French theoretical physicist and talented engineer Sadi Carnot tried to find an answer to this question. In 1824, his theoretical accounts of the processes taking place in gases were made public.
The main idea embedded in an ideal machine can be considered as carrying out reversible processes with an ideal gas. We start with the gas expansion isothermally at temperature T1. The amount of heat required for this is Q1. After The gas without heat transfer expands (adiabatic process). Having reached the temperature T2, the gas is isothermally compressed, transferring the energy Q2. The gas is returned to its original state adiabatically.
КПД идеального теплового двигателя Карно при accurate calculation is equal to the ratio of the temperature difference between the heating and cooling devices to the temperature that the heater has. It looks like this: η = (T1 - T2) / T1.
The possible efficiency of a thermal machine, the formula of which has the form: η = 1 - T2/ T1, depends only on the values of the temperatures of the heater and the cooler and can not be more than 100%.
Moreover, this relationship allows us to prove that the efficiency of thermal machines can be equal to unity only when the refrigerator reaches absolute zero temperatures. As you know, this value is unattainable.
Theoretical calculations of Carnot allow us to determine the maximum efficiency of a thermal machine of any design.
The theorem proved by Carnot reads as follows.An arbitrary thermal machine under any conditions is not capable of having an efficiency greater than that of an ideal heat engine.

Example 1. What is the efficiency of an ideal thermal machine, in the case that the temperature of the heater is 800aboutWith, and temperature of a refrigerator on 500aboutFrom below?
T1= 800aboutC = 1073 K, ΔT = 500aboutC = 500 K, η -?
Decision:
By definition: η = (T1 - T2) / T1.
We are not given the temperature of the refrigerator, but ΔT = (T1 - T2), from here:
η = ΔT / T1 = 500 K / 1073 K = 0.46.
Answer: Efficiency = 46%.
Example 2. Определите КПД идеальной тепловой машины, если за the account of the purchased one kilojoule of heater energy makes a useful work of 650 J. What is the temperature of the heater of the heat engine, if the temperature of the cooler is 400 K?
TO1 = 1 kJ = 1000 J, A = 650 J, T2 = 400 K, η -?, T1 =?
Decision:
In this problem we are talking about a thermal installation, the efficiency of which can be calculated by the formula:
η = A / Q1.
To determine the temperature of the heater, we use the efficiency formula of an ideal heat engine:
η = (T1 - T2) / T1 = 1 - T2/ T1.
Performing mathematical transformations, we get:
T1 = T2 / (1- η).
T1 = T2 / (1- A / Q1).
Calculate:
η = 650 J / 1000 J = 0.65.
T1 = 400 K / (1- 650 J / 1000 J) = 1142.8 K.
Answer: η = 65%, T1 = 1142.8 K.
The ideal heat engine is designed for perfect processes. Work is done only in isothermal processes, its value is defined as the area bounded by the Carnot cycle graph.

In fact, to create conditions for the flowthe process of changing the state of a gas without the accompanying temperature changes is impossible. There are no materials that would eliminate heat exchange with surrounding objects. The adiabatic process becomes impossible. In the case of heat exchange, the gas temperature must change.
Efficiency of heat engines created in realconditions are significantly different from the efficiency of ideal engines. Note that the processes in real engines occur so quickly that the variation of the internal heat energy of the working substance in the process of changing its volume cannot be compensated for by the inflow of heat from the heater and return to the refrigerator.
Real engines work on other cycles:
Create equilibrium processes in realengines (to bring them closer to the ideal) in terms of modern technology is not possible. The efficiency of heat engines is much lower, even taking into account the same temperature conditions as in an ideal thermal installation.
But you should not reduce the role of the calculation formula for the efficiency of the Carnot cycle, since it becomes the starting point in the process of working to increase the efficiency of real engines.
Проводя сравнение идеальных и реальных тепловых engines, it is worth noting that the temperature of the refrigerator of the latter can not be any. Usually the refrigerator is considered an atmosphere To take the temperature of the atmosphere is possible only in approximate calculations. Experience shows that the temperature of the cooler is equal to the temperature of exhaust gases in engines, as is the case in internal combustion engines (for short, ICE).

ICE - the most common in our worldheat engine. The efficiency of the heat engine in this case depends on the temperature created by the burning fuel. A significant difference of ICE from steam engines is the fusion of the functions of the heater and the working fluid of the device in the air-fuel mixture. Burning the mixture creates pressure on the moving parts of the engine.
Rising temperatures of the working gases reachsignificantly changing the properties of the fuel. Unfortunately, it is impossible to do this indefinitely. Any material from which the engine combustion chamber is made has its melting point. Heat resistance of such materials is the main characteristic of the engine, as well as the ability to significantly affect the efficiency.
If we consider the steam turbine, the temperatureThe working steam at the inlet of which is 800 K, and the exhaust gas is 300 K, then the efficiency of this machine is 62%. In reality, this value does not exceed 40%. This reduction is due to heat loss when the turbine casing is heated.

The highest efficiency value of internal enginescombustion does not exceed 44%. Increasing this value is a matter of the near future. Changing the properties of materials and fuels is a problem on which the best minds of mankind work.


























