
Let's talk about what is OVR in inorganic and organic synthesis.
Redox reactions include those processes that will result in a change in the degree of oxidation in two or more chemical elements in complex or simple substances.

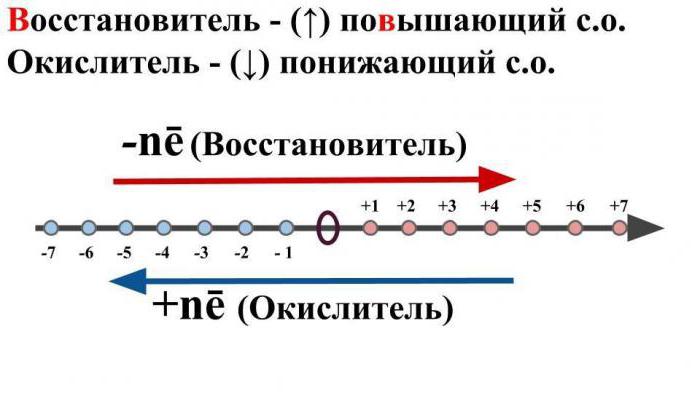
By oxidation is meant such a chemicala reaction in which an atom or a specific ion gives off electrons, while lowering its initial oxidation state. This process is typical for metals.
By the process of restoration is meantchemical transformation, which will result in a decrease in the degree of oxidation in the ion or a simple substance, with the addition of electrons. This reaction is characteristic for nonmetals and acid residues.

Considering the question of what is OVR, one can not ignore such a notion as a "reductant".
By it is meant a neutral molecule orcharged ion, which as a result of chemical interaction will give the other ion or atom electron, while increasing its degree of oxidation.

Arguing over what is OVR is also importantmention also a term such as "oxidizer". By this, it is customary to mean such ions or neutral atoms that, during chemical interaction, will take negative electrons from other atoms or neutral particles. At the same time, its initial oxidation state will decrease.
Arguing over what is OVR, it is necessary to note about those varieties of these processes, which are most often considered in inorganic and organic synthesis.
Intermolecular interactions assume suchprocesses in which the atoms of both the reducing agent and the oxidizer are located in different starting materials that enter into interaction. An example of this type of transformation is the interaction between manganese oxide (4) and hydrochloric acid, which results in gaseous chlorine, divalent manganese chloride, and water.
In this chemical process, aschlorine anions appear oxidizing as they interact. The cation of manganese (with oxidation state +4) exhibits oxidative abilities in the reaction, taking two electrons, is restored.
The intramolecular interaction issuch chemical transformations, insofar as both the atoms of the reducing agent and the atoms of the oxidizer are initially one source substance, and after the completion of the transformation they are in various reaction products.
As an example of this type of reaction,to represent the decomposition of potassium chlorate. When heated, this substance will turn into potassium chloride and oxygen. Oxidizing properties will be characteristic for the chlorate anion, which, taking five electrons in the reaction, will be reduced to chloride.
In this case, the oxygen anion will manifestreducing properties, oxidizing to molecular oxygen. So what is OVR in this case? This is the process of transfer of electrons between ions, leading to the formation of two reaction products.
Also to this type of chemical transformation,occurring with a change in the degrees of oxidation of elements originally found in one formula, is the process of decomposition of ammonium nitrite. Nitrogen, standing in the ammonium cation, having a degree of oxidation -3, during the process gives up six electrons and is oxidized to molecular nitrogen. And that nitrogen, which is part of the nitrite, takes six electrons, while it is a reducing agent, and during the reaction it is oxidized.
What is OVR in chemistry? The definition considered above shows that these are transformations associated with changes in several elements of the oxidation states.
Самоокисление и восстановление (диспропорция) assumes such processes, in the course of which, as a reducing agent and an oxidizer, there is one initial atom that will increase, and simultaneously reduce its oxidation state after completion of the interaction. Arguing over what is OVR in chemistry, examples of such transformations can be found even in the course of chemistry in the secondary school. The decomposition of potassium sulfite upon heating leads to the formation of two salts of this metal: sulphide and sulfate. Sulfur with an oxidation state of +4 shows both reducing and oxidizing properties, increasing and decreasing the oxidation state.
To understand what OVI means in chemistry, let's call itanother type of such chemical transformations. Counterproportion involves such processes, as a result of which the atoms of the reducing agent and oxidizer are in the composition of different initial components, but on the right side they form one reaction product. For example, in the interaction of sulfur oxide (4) with hydrogen sulphide, sulfur and water will form. A sulfur ion with an oxidation state of +4 will take four electrons, and a sulfur ion with an exponent -2 will lose two electrons. As a result, they both turn into a simple substance, in which the degree of oxidation is zero.

Considering the question of what is OVR in chemistry,we note that these are numerous transformations, through which live organisms function, various natural processes and phenomena occur. In order to arrange the coefficients in such equations, it is necessary to compile an electronic balance.


























